Abstract
Background: Asciminib is the first tyrosine kinase inhibitor (TKI) to Specifically Target the ABL Myristoyl Pocket (STAMP). In the ASCEMBL Phase 3 randomised study comparing asciminib to bosutinib in patients with CML in chronic phase (CP) after ³2 prior TKIs, asciminib demonstrated superior efficacy and tolerability vs bosutinib (major molecular response (MMR) rate at week 96: 37.6% vs 15.8%, treatment discontinuation 7.7% vs 26.3%) (Rea et al, EHA 2022). Here we present the real-world clinical outcomes of TKI-resistant/ intolerant CML patients in Australia who received asciminib through a MAP approved by Novartis.
Methods: All haematologists who had requested asciminib through MAP were approached to contribute data on the clinical outcomes of their CML patients. The database captured details on patient demographics, previous TKI use, reason for asciminib use (lack of efficacy vs intolerance), adverse events and efficacy based on BCR::ABL1 transcript levels.
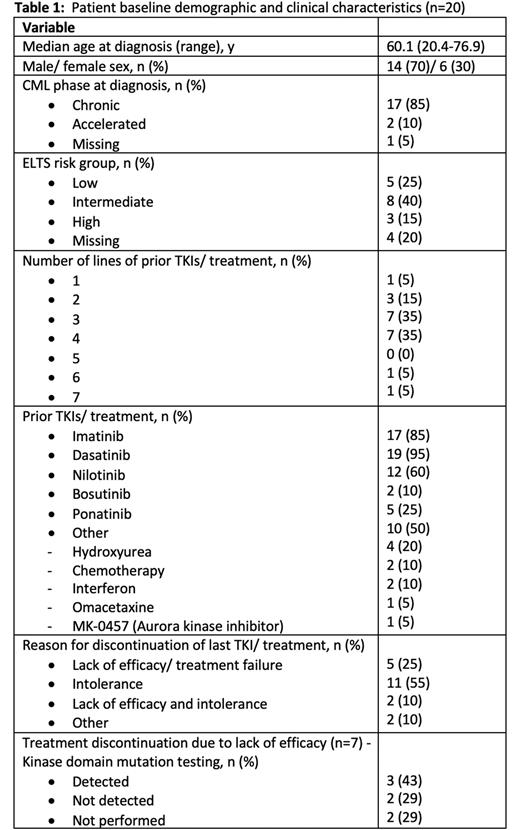
Results: At data cut-off for analysis on 12 July 2022, 20 patients had data available for analysis. Table 1 summarises the baseline patient characteristics. Median age of the patients was 60.1 years at time of diagnosis and most patients were alive at time of survey (n=16, 80%, n=1 unknown). Comorbidities were present in 90% of patients, the most prevalent being cardiovascular (75%), respiratory (30%) and endocrine (30%) conditions.
The median time from diagnosis to asciminib commencement was 9.5 years (range 1.5-19.8) with most patients receiving asciminib as 4th (n=7) or 5th line (n=7) of treatment. Median BCR::ABL1IS at time of asciminib commencement was 1.05% (range 0-337). 11 (55%) patients discontinued their last TKI due to intolerance, 5 (25%) due to lack of efficacy and 5 (25%) due to both intolerance and lack of efficacy. The starting dose of asciminib was 40mg twice daily in 15 (75%) patients, 200mg twice daily in 3 (15%) patients and 1 (5%) patient each received 20mg twice daily and 80mg daily dosing. For the 7 (35%) patients who had commenced asciminib due to lack of efficacy (± intolerance), 5 patients had kinase domain (KD) mutation testing performed - T315I (n=2), V299L (n=1) and no mutations (n=2) were detected.
Of the 20 patients, 5 (25%) were already in MMR (BCR::ABL1IS ≤0.1%) at commencement of asciminib and 9 (45%) achieved MMR at a median duration of 2.2 months (range 1.2-7.7). Of the 14 patients who achieved/ maintained MMR, 9 (64%) achieved MR4 (BCR::ABL1IS ≤0.01%) at a median duration of 2.6 months (range 1.3-24.1) while 3 (21%) were already in MR4 (n=2 unknown). Six (30%) patients failed to achieve MMR, of whom only 1 patient achieved BCR::ABL1IS ≤1% at 7.8 months; 4 patients had treatment failure to their last TKI (asciminib ≥4th line). One other patient with treatment resistance due to V299L mutation achieved MR4 on asciminib with sustained response.
In the ponatinib pre-treated group (n=5), all 3 patients who commenced asciminib (200mg twice daily) for treatment failure to last TKI (n=2 T315I mutation) discontinued treatment (progressive disease (n=2), unknown (n=1, lost to follow-up)). Two other patients (ponatinib intolerance (n=1) and ponatinib bridging for T315I mutation (n=1)) achieved and maintained MR4 responses to asciminib.
14 (70%) patients were still receiving asciminib at time of analysis for a median duration of 13.4 months (range 0.4-45.2). Only 1 patient required a dose reduction due to Grade 1 myalgia. Reasons for discontinuation in 6 patients were intolerance (nausea) (n=1), unclear (n=1, lost to follow-up), progression to accelerated phase disease and received an allogeneic stem cell transplant (n=1) and death (n=3) - 2 from non-CML related causes and 1 patient from CML blast crisis. This patient had T315I mutation detected after 1st line imatinib and received asciminib as his 8th line of treatment. One patient recommenced asciminib through MAP after failure of second treatment-free remission which had lasted for 3.2 years (BCR::ABL1IS 0.16%); MMR/MR4 was regained within 3 weeks of re-commencement.
Conclusion: In this heavily pre-treated population of CML patients most of whom had underlying medical comorbidities, asciminib was generally well-tolerated and demonstrated excellent responses more so in favour of the TKI-intolerant patients, which are associated with improved long-term outcomes. These results validate the role of asciminib as a useful novel CML treatment.
Disclosures
Chee:Otsuka: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees. Grigg:Novartis: Membership on an entity's Board of Directors or advisory committees. Szer:CSL: Consultancy, Honoraria, Speakers Bureau; Biocryst: Consultancy, Honoraria, Speakers Bureau; Takeda Pharmaceuticals: Consultancy, Honoraria, Speakers Bureau; Alexion: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Eli-Lilly: Consultancy, Honoraria, Speakers Bureau; Sobi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Yeung:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria; Pfizer: Honoraria; BMS: Honoraria, Research Funding. Hughes:Novartis: Consultancy, Research Funding; Enliven: Consultancy, Research Funding; BMS: Consultancy, Research Funding. Shanmuganathan:Amgen: Other: Meeting sponsorship; Novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal